Feature
Feature
A Standalone Viewer for Browsing eCTD Submissions
A standalone eCTD viewer application for Windows PCs, fully compliant with PMDA, FDA, and EMA eCTD regulatory requirements.
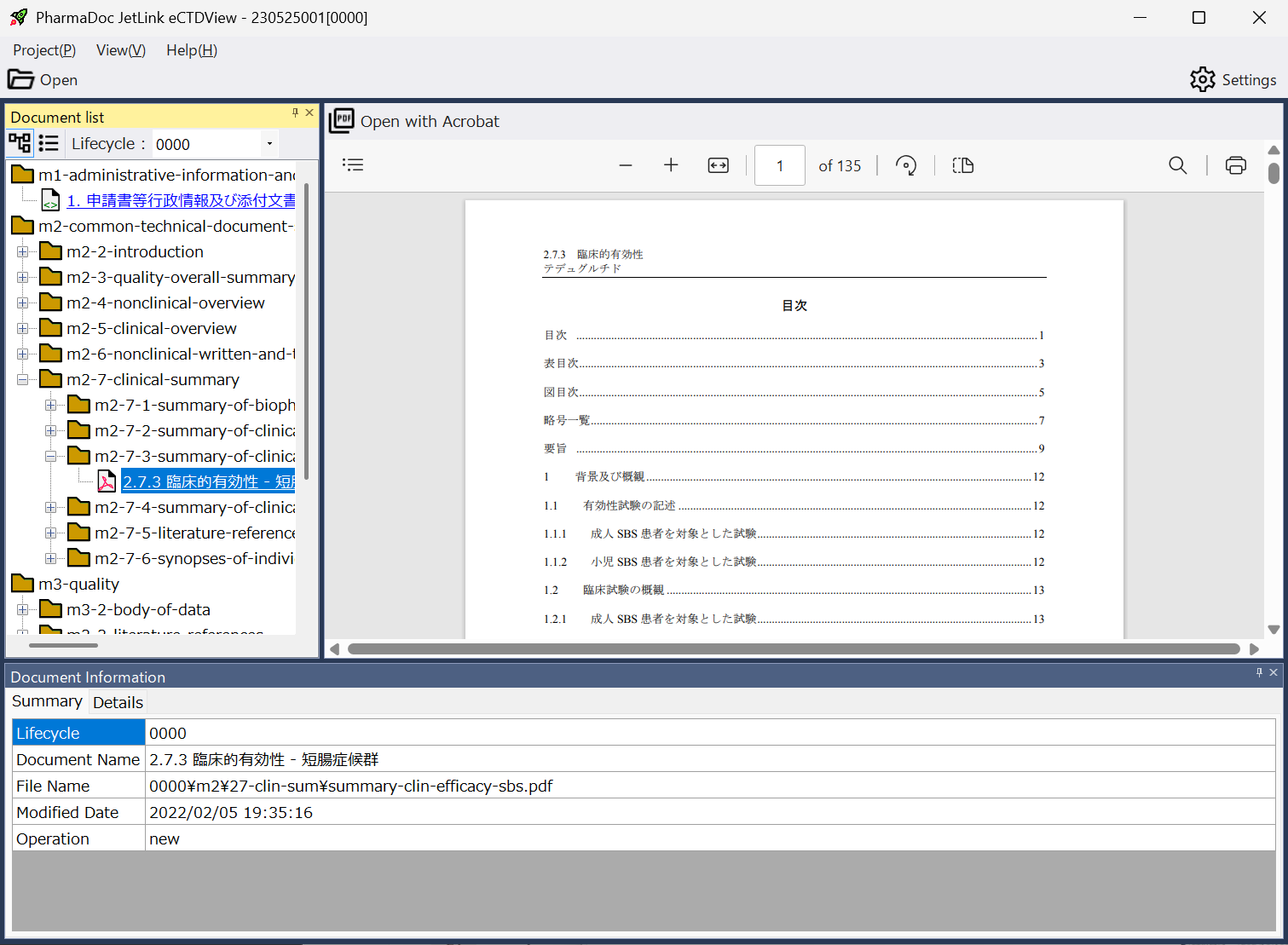
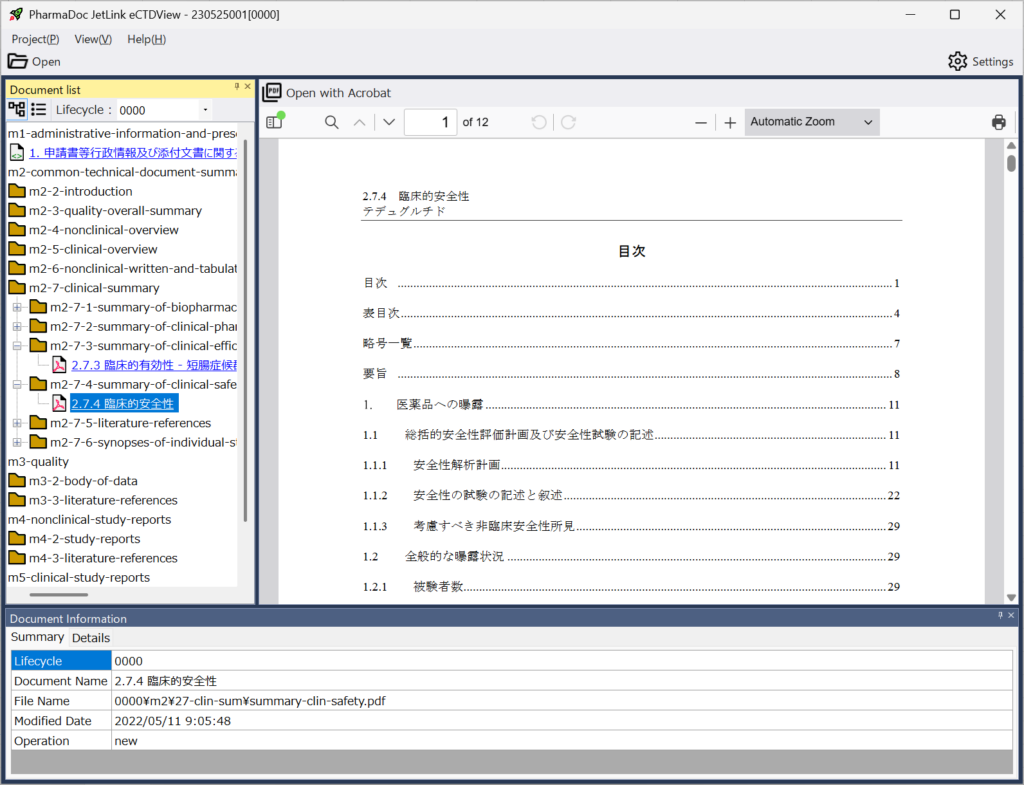
View Detailed Information Contained in eCTD Packages
The application allows you to view a wide range of information, including:
- List of leaf files for each lifecycle
- Metadata for each leaf file:
-- Document title
-- PDF modification date
-- CTD section number
-- Additional keywords (e.g., study number, document type)
-- Information on leaf files before replacement
- Preview of PDF leaf files (available in the paid version only)
By importing the latest Controlled Vocabulary (CV) files distributed by ICH and regulatory authorities, you can display up-to-date keyword information.
Supports Both eCTD v3.2 and v4.0
PharmaDoc JetLink eCTDView supports both eCTD v3.2 and eCTD v4.0 formats.
A Free Version You Can Use Indefinitely
A free, non-expiring version is available for immediate download here .
We also offer a time-limited evaluation version of the paid edition .
For inquiries regarding the paid version, please contact our support team.
Feature Comparison Within the PharmaDoc JetLink Series
PharmaDoc JetLink eCTDView is a subset of the functionality provided by PharmaDoc JetLink, a document interlinking tool, restructured specifically as an eCTD viewer.
| PharmaDoc JetLink eCTDView | PharmaDoc JetLink | ||
|---|---|---|---|
| Free Ver. | Paid Ver. | ||
| Viewing eCTD Information | |||
| File name | 〇 | 〇 | 〇 |
| Document title | 〇 | 〇 | 〇 |
| File modification date | 〇 | 〇 | 〇 |
| CTD section number | 〇 | 〇 | 〇 |
| Additional keywords (e.g., study number) | 〇 | 〇 | 〇 |
| Replacement information | 〇 | 〇 | 〇 |
| Viewing PDF Leaf Files | |||
| Preview within the application | - | 〇 | 〇 |
| Open in Acrobat | - | 〇 | 〇 |
| Editing Inter-Document Links | |||
| Create link annotations | - | - | 〇 |
| Assign link destinations | - | - | 〇 |
| Updating eCTD Backbone XML | |||
| Pseudo-replace functionality | - | - | 〇 |
| Update file modification dates | - | - | 〇 |
Contact us for product introduction and consultation!
Please feel free to contact us.